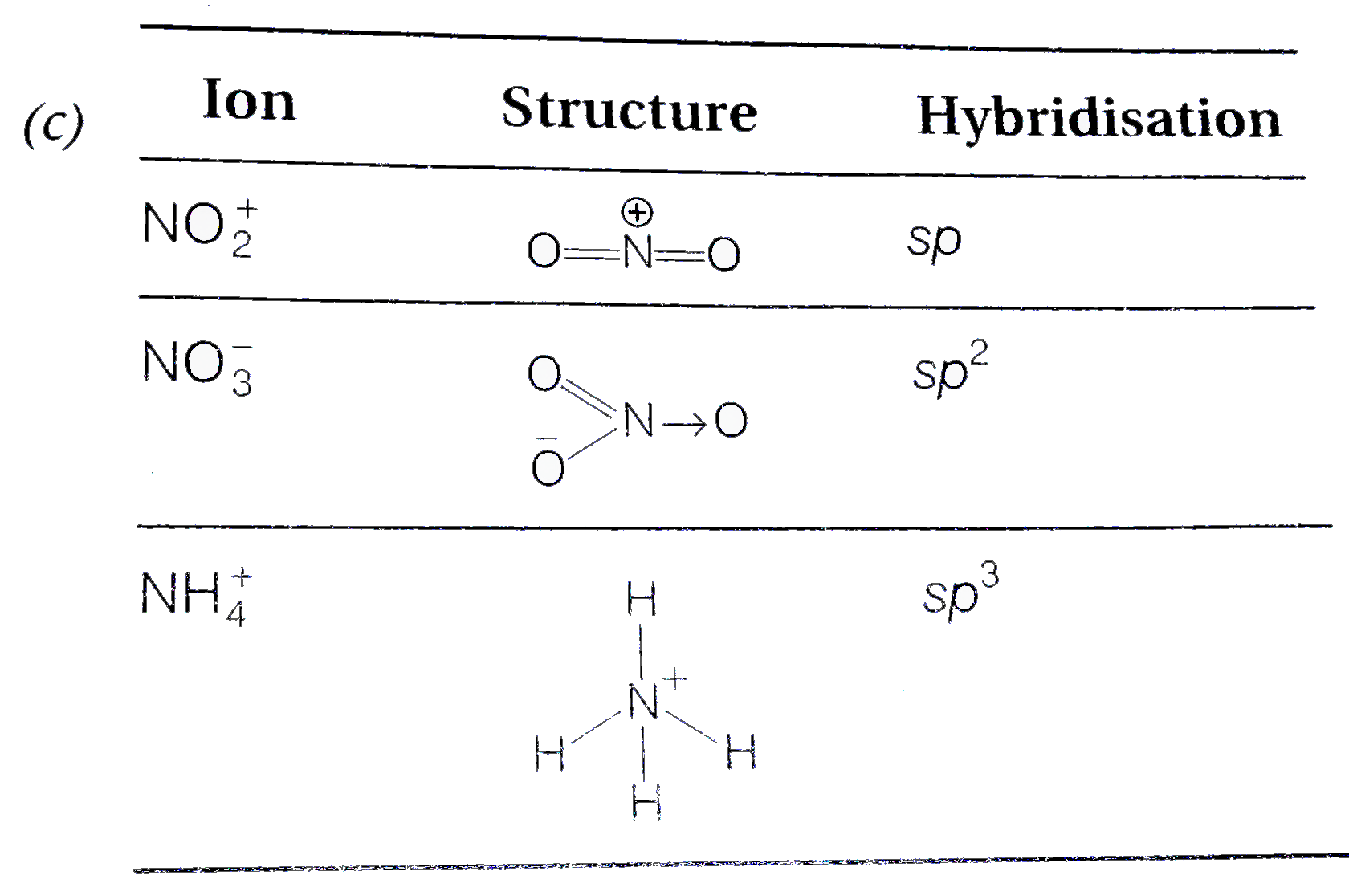
The species in which the N-atom is in a state of sp hybridisation is (a) NO2^- (b) NO3^- (c) NO2 (d) NO2^+ - Sarthaks eConnect | Largest Online Education Community

Bond Angle And Hybridization Of NO2,NO2-,NO2+,NO3-||Lewis Dot Structure||iit,neet,cbse, icse,kvpy|| - YouTube

NO2- lewis structure, molecular geometry, bond angle, hybridization | Molecular geometry, Molecular, Molecular shapes
How to find the hybridizationof[Ni(CN)4]2 n ntand the d orbital used in it. What is the hybridization of NO2?
The hybridization of atomic orbitals of nitrogen in NO2^+ , NO^-2 and NH4^+ are - Sarthaks eConnect | Largest Online Education Community






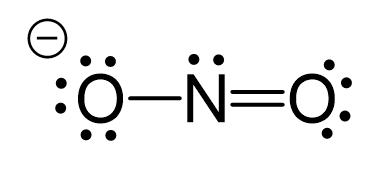


![What is the hybridization in [Pt(h2o)2(no2)cl]br complex ?? - askIITians What is the hybridization in [Pt(h2o)2(no2)cl]br complex ?? - askIITians](https://files.askiitians.com/cdn1/question-images/185861-15057572561371714211946.jpg)