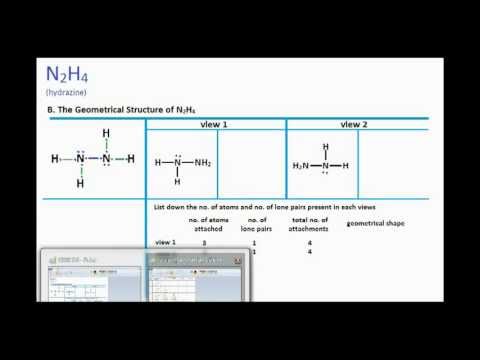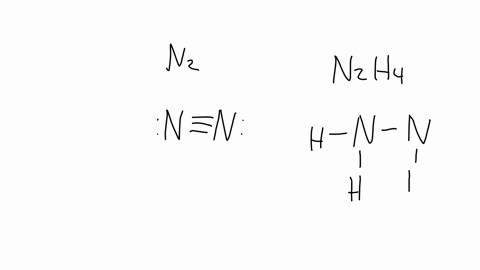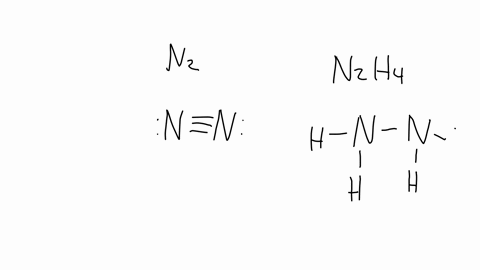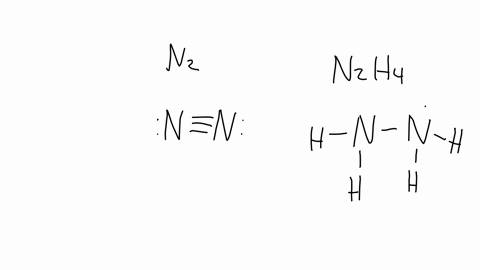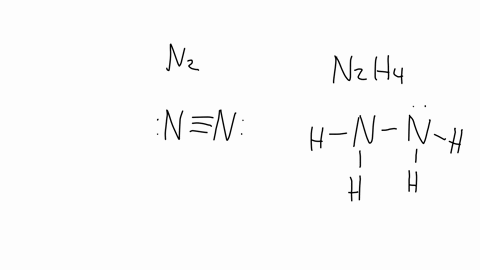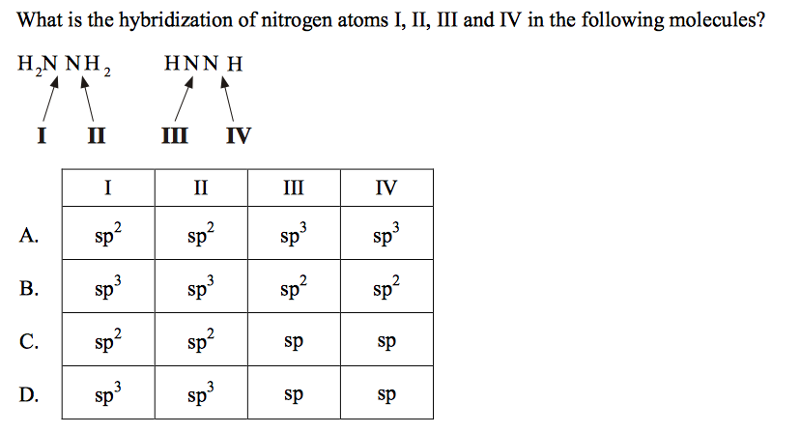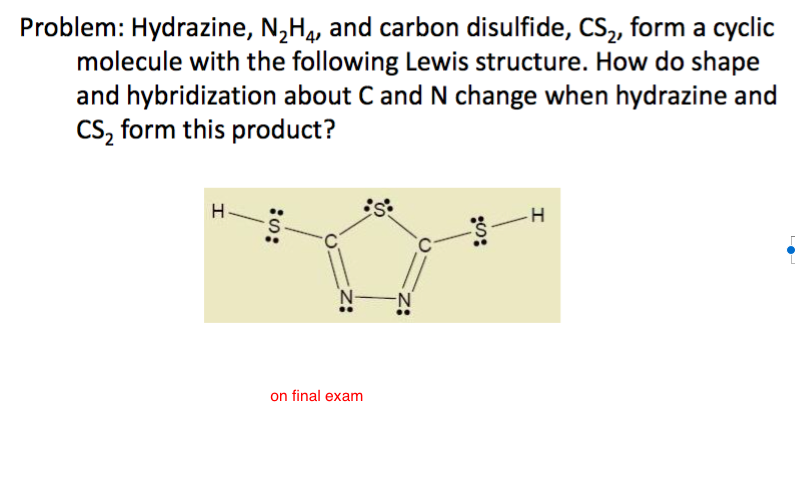
In ${{N}_{2}}{{H}_{4}}$ molecule type of overlapping present between N-H bond:- (A) $s-p$(B) $s{{p}^{2}}-p$(C) $s{{p}^{3}}-s$ (D) $s{{p}^{2}}-s$

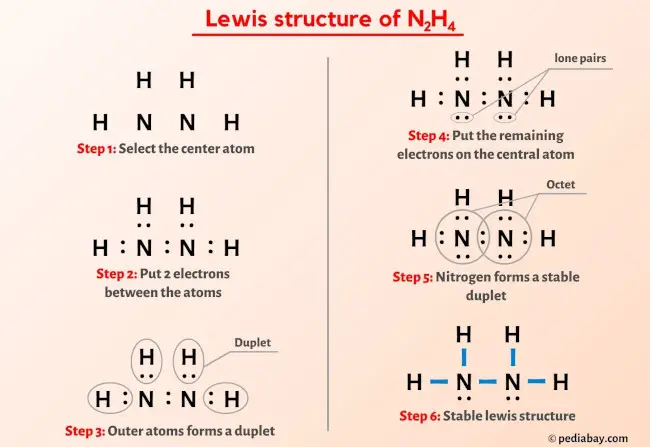
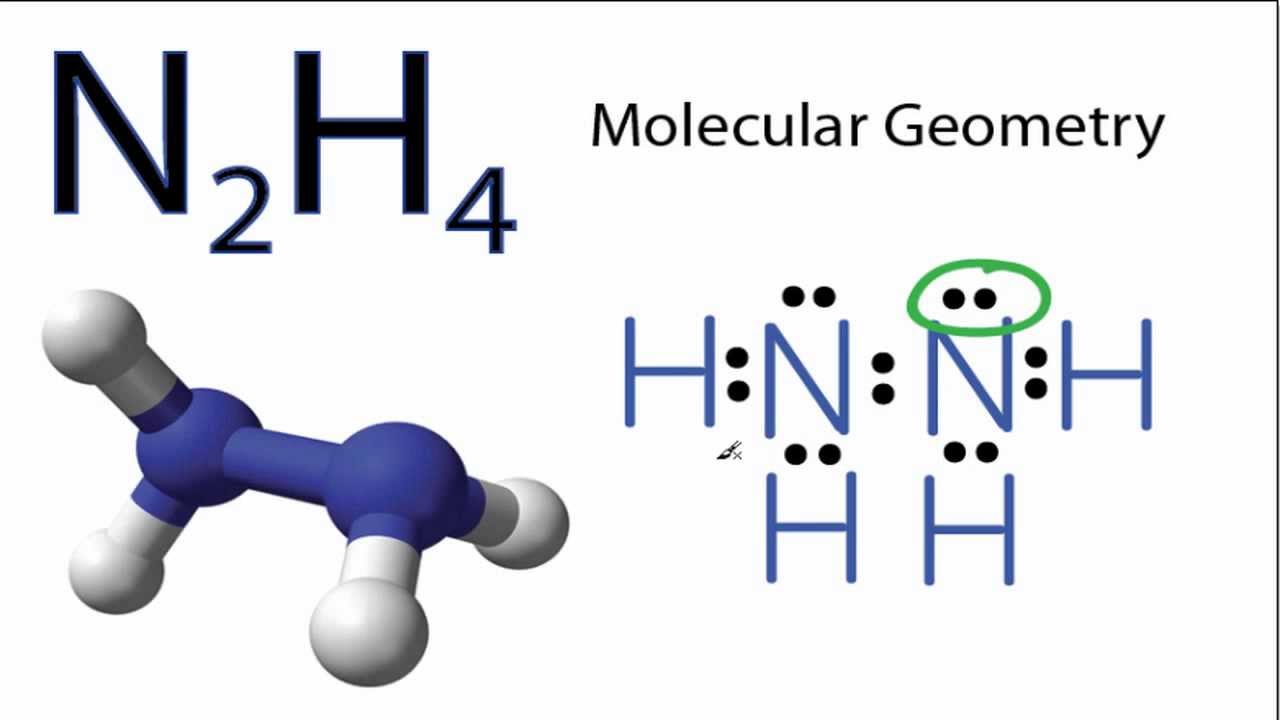
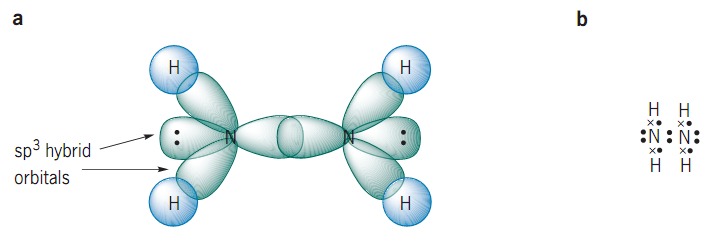
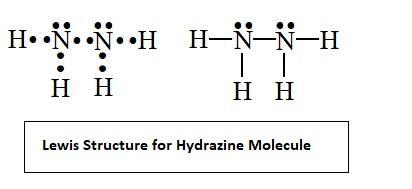
SOLVED: The nitrogen atoms in N2 participate in multiple bonding, whereas those in hydrazine, N2H4, do not. Draw Lewis structures for both molecules. What is the hybridization of nitrogen atoms in each

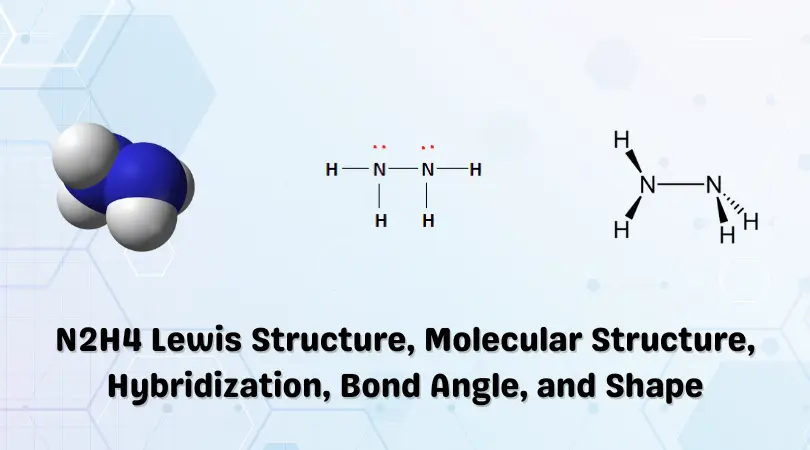
Hybridisation of n2h4 - Chemistry - Chemical Bonding and Molecular Structure - 13024745 | Meritnation.com