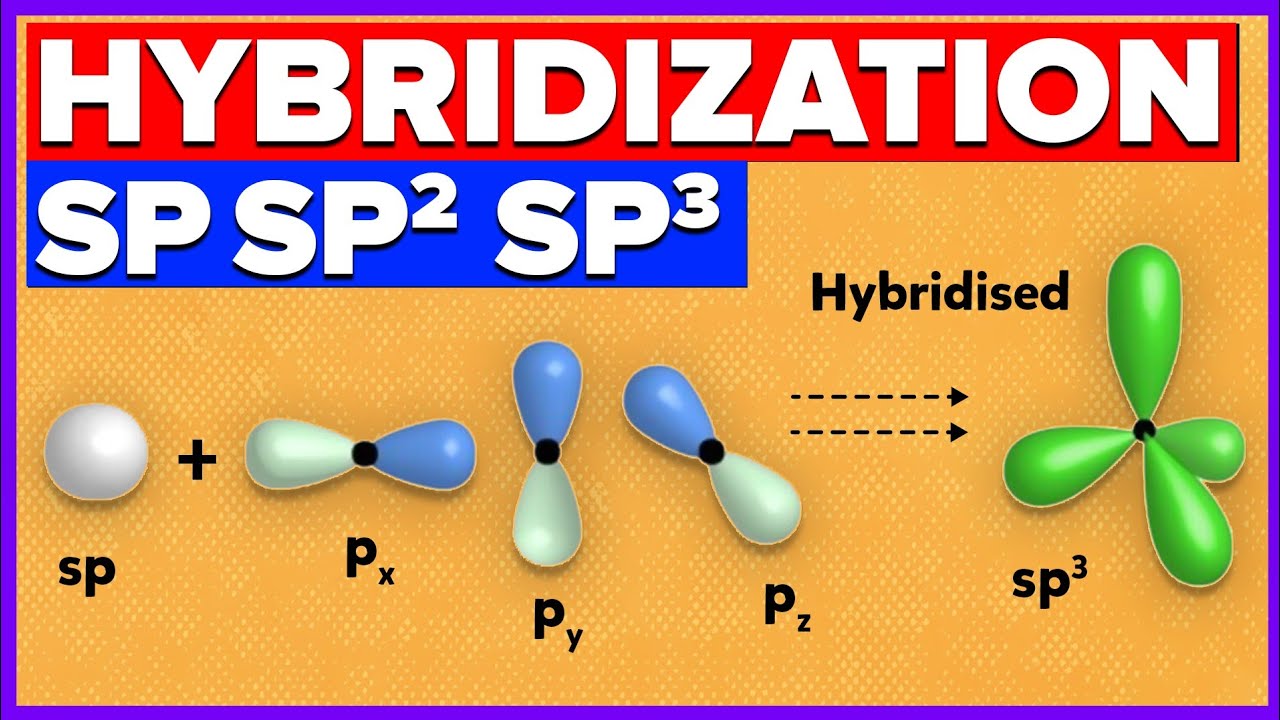
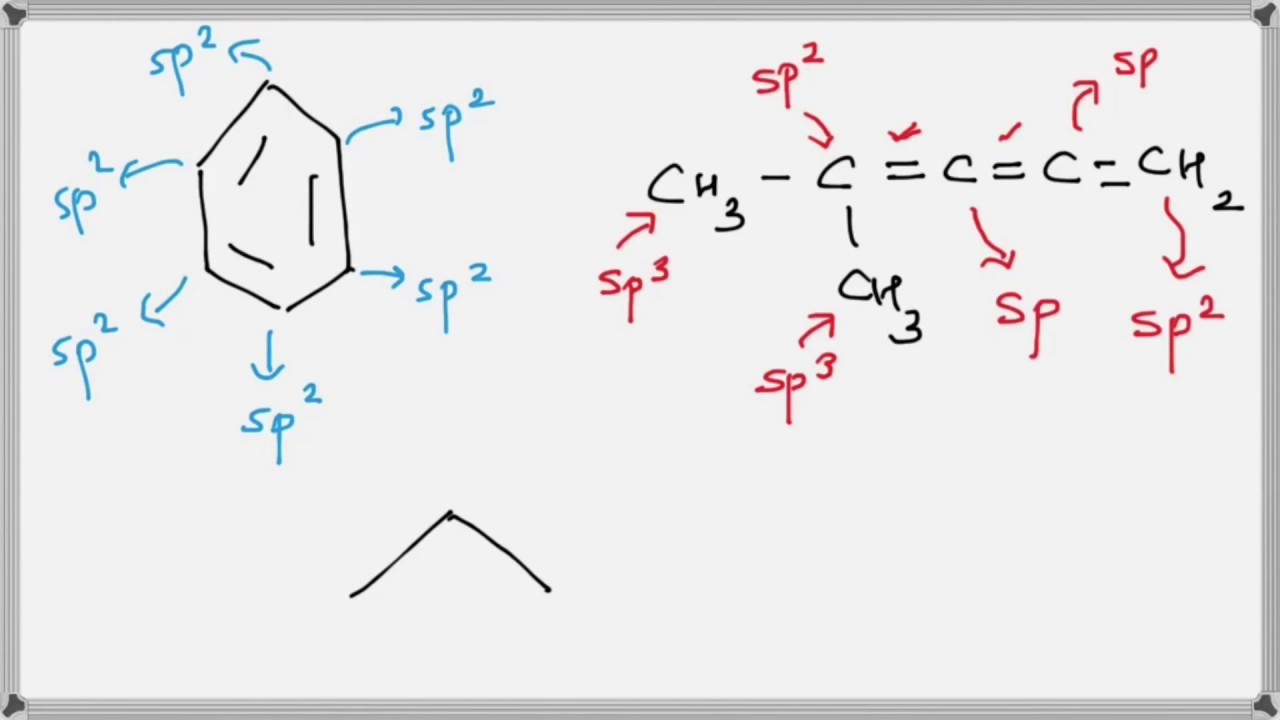
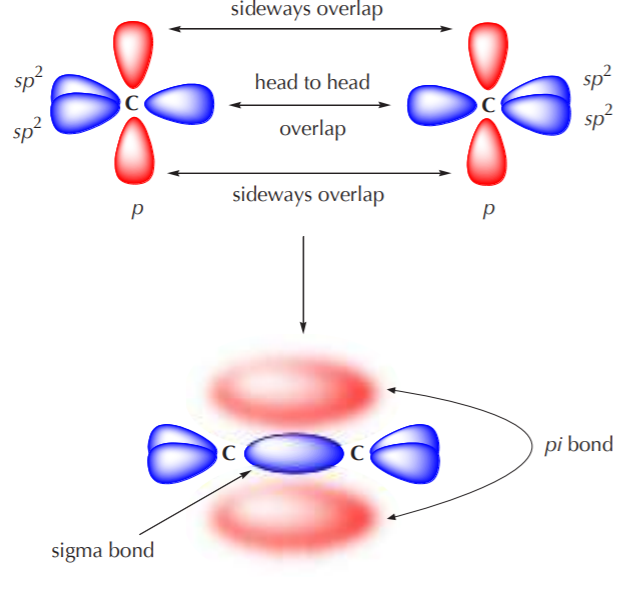
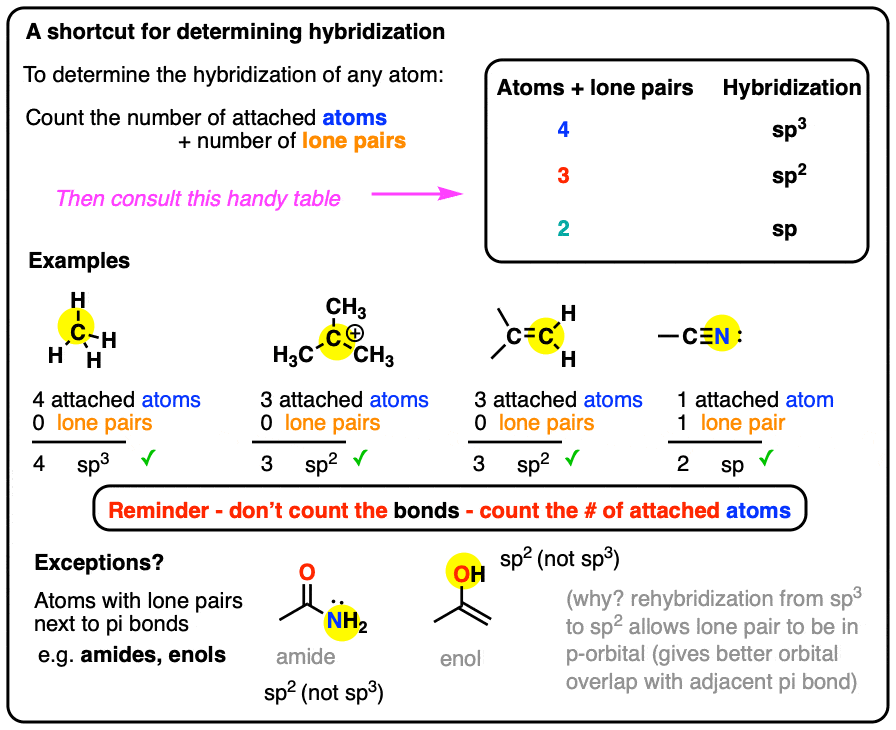
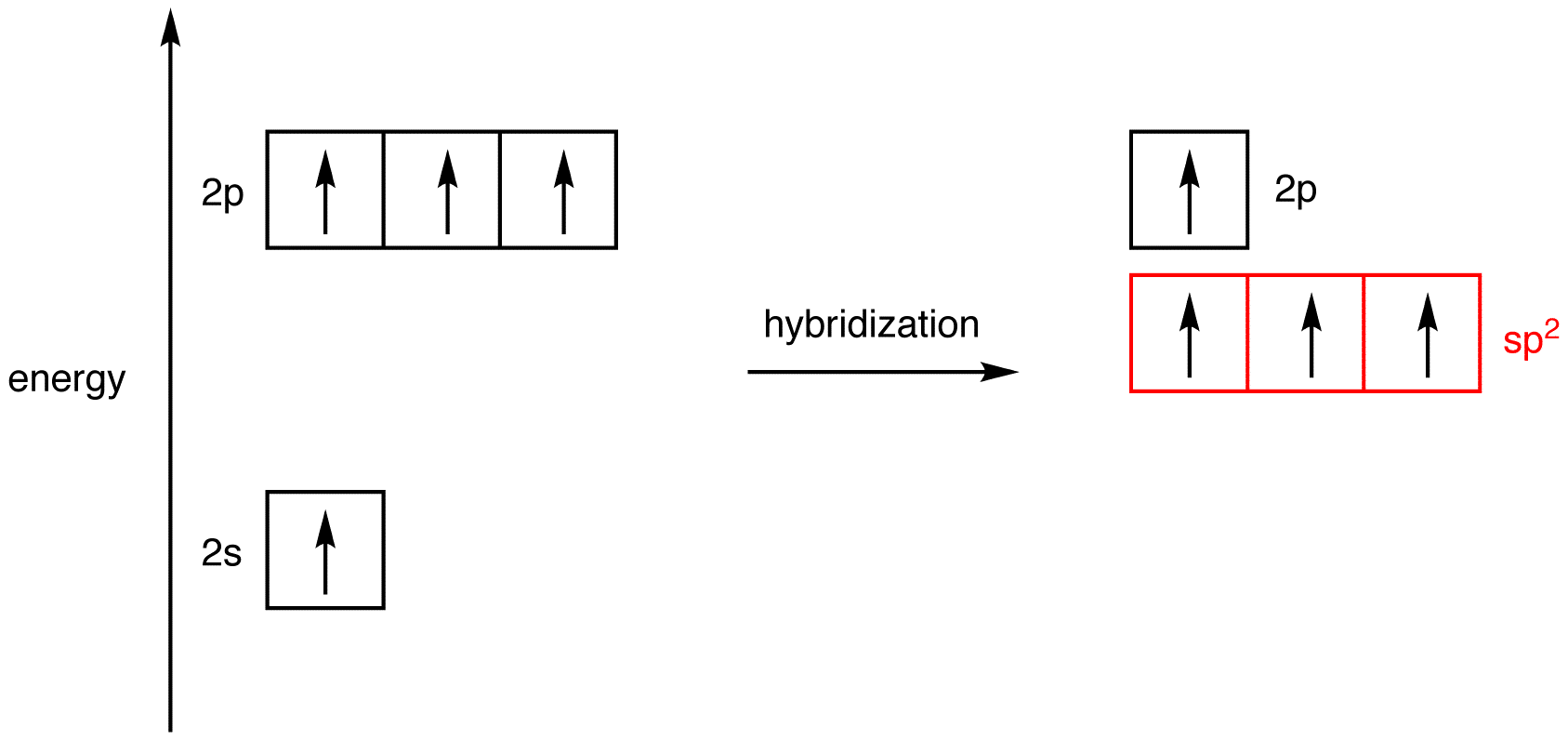
a Atomic C-hybridization. b C hybridization in carbon-based materials.... | Download Scientific Diagram

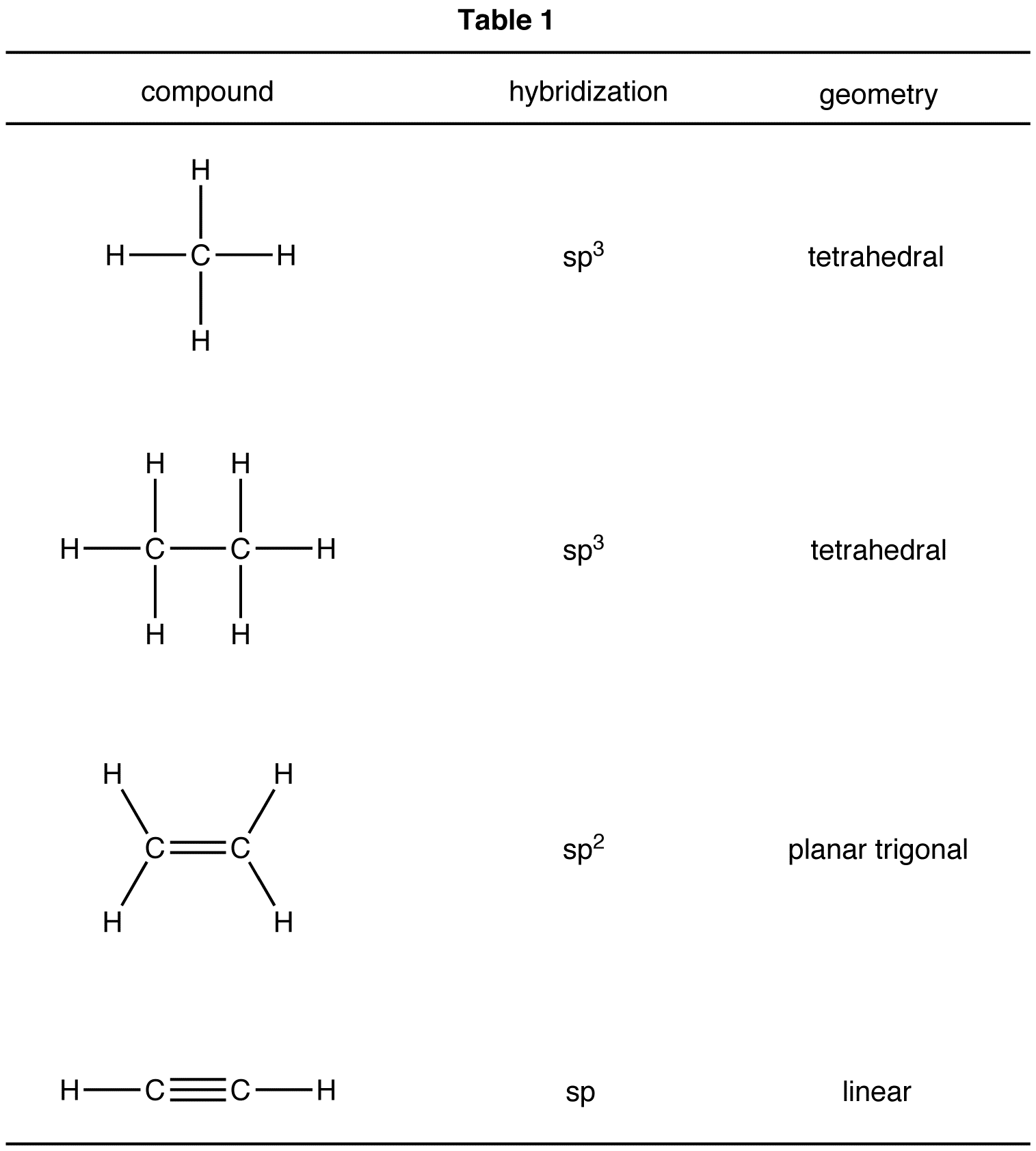
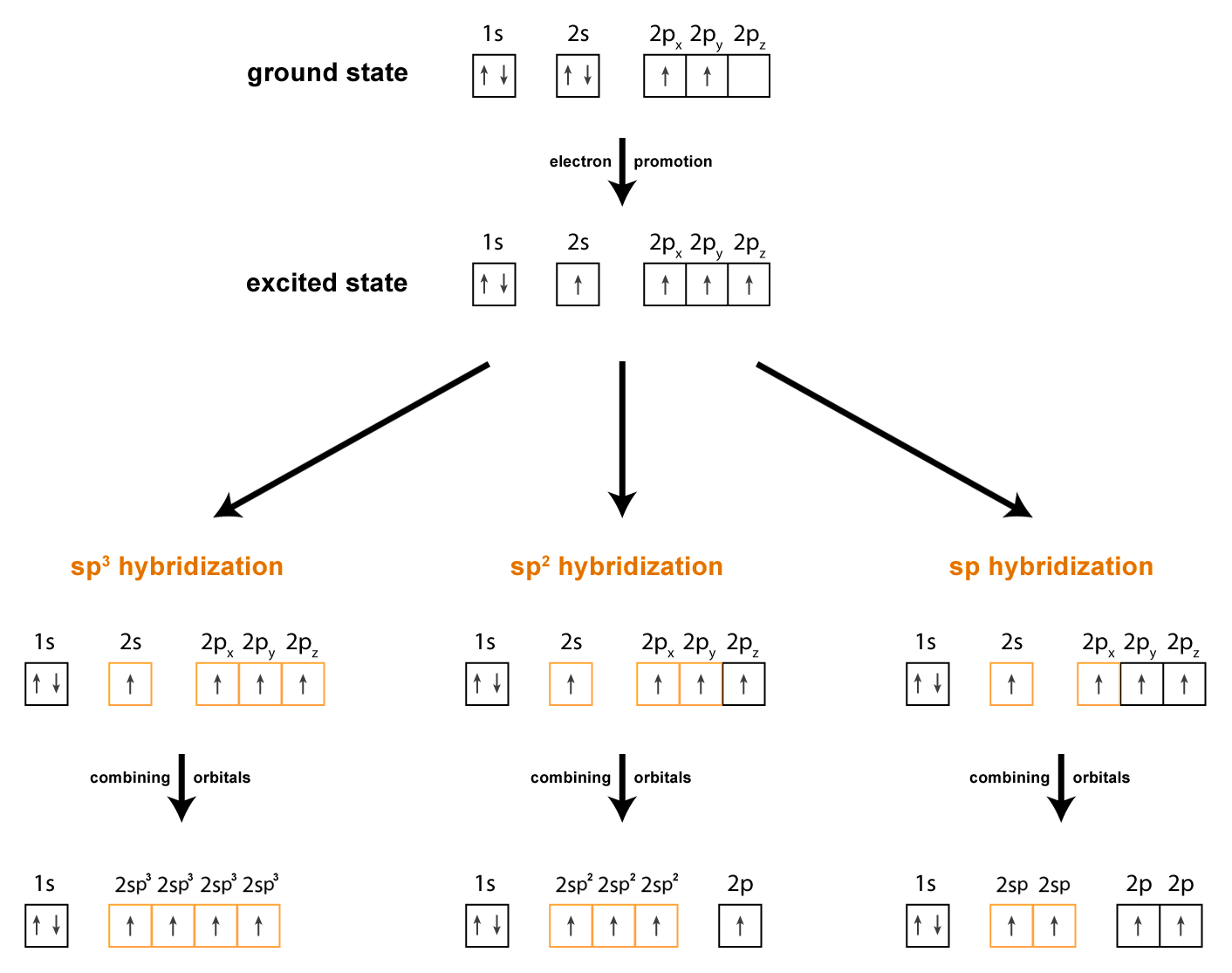
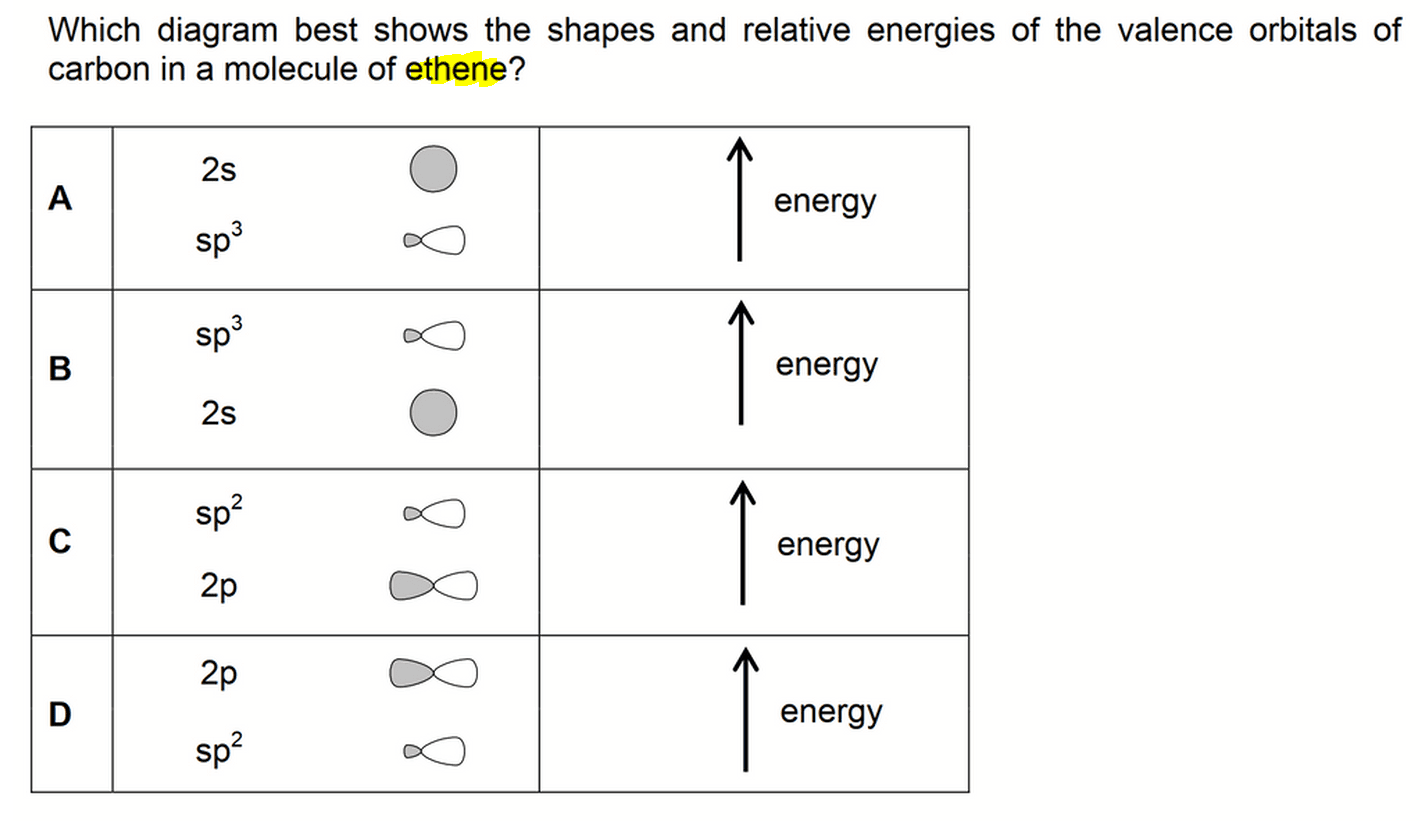
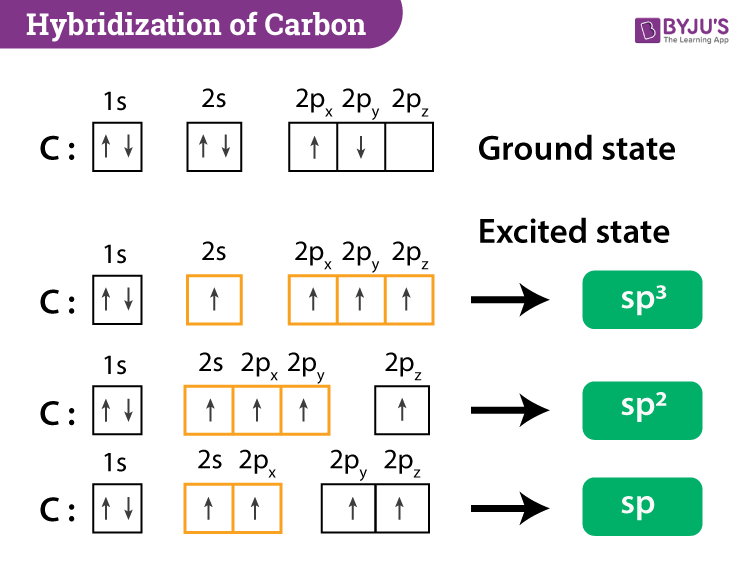
What is the geometry of: (a) an sp3 hybridized carbon atom? (b) an sp2 hybridized carbon atom? (c) an sp hybridized carbon atom? (d) a trivalent nitrogen atom (three bonds on the

Classification of Negative Charge Discriminate Hybridization with Aromatic and Anti-aromatic Behavior of Organic Compounds - Innovative Mnemonics
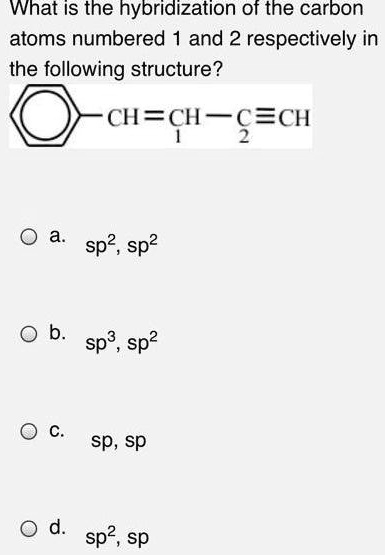
SOLVED: What is the hybridization of the carbon atoms numbered 1 and 2, respectively, in the following structure? CH=CH F FcH sp2, sp2 sp3, sp2 sp, sp d. sp2, sp